Answers
Answer;
New Pressure of Gas = 7.33 kPa
Explanation:
Given the initial and final volume occupied by a sample of a gas at constant temperature, we want to get the final pressure of the gas given the initial pressure value
To answer this, we look for the gas law that links voume and pressure at constant temperature
The gas law here is Boyle's law
It states that volume and pressure are inversely proportional. So we would expect a volume decrease where there is a pressure increase and vice versa
Mathematically, we have this as:
\(P_iV_i=P_fV_f\)where i represents the initial values and f represents the final values
What we are looking for is the final pressure so, we rewrite the formula above as follows:
\(P_f\text{ = }\frac{P_iV_i}{V_f}\)Lastly, we go on to substitute the given values
Let us have a look at the volume units
It must be understood that we should have same unit
Since 1000 mL = 1L
Then 336.33 mL = 0.33633 L
Finally, we substitute as follows:
\(P_f\text{ = }\frac{25.72\times0.33633}{1.18}\text{ = 7.33 kPa}\)Related Questions
3. Give the n and I values for the following orbitals
a. 1s
b. 3s
c. 2p
d. 4d
e. 5f
Answers
Answer: 1s-n=1,l=0
3s-n=3,l=0
2p-n=2,l=1
4d-n=4,l=2
5f-n=5,l=3
Explanation: An electron has 4 quantum numbers .
n-principal quantum number (explains about the shell)
and has values 1,2,3....
l-angular quantum number (shape of the orbital)
and is described by shapes spherical (l=0),polar (l=1),clover leaf (l=2)
l=0(s subshell)
l=1(p subshell)
l=2(d subshell)
l=3(f subshell)
There can be no zero for the main quantum number (n). Therefore, the permitted values for n are 1, 2, 3, 4, and so forth.
Any integer between 0 and n – 1 can serve as the angular quantum number (l). For instance, if n = 3, l can be one of 0, 1, or 2.
Answer:
Explanation:
The n (Principal Quantum Number) and l (Azimuthal Quantum Number) values for the following orbitals are:
a)1s: n=1 , l =0
b) 3s: n=3 , l =0
c)2p: n=2 , l =1
d)4d: n=4 , l =2
e)5f: n=5 , l =3
In any orbital there are four different quantum numbers i.e.,
•Principal Quantum Number
•Azimuthal Quantum Number
•Magnetic Quantum Number
• Spin Quantum Number
Principal Quantum Number tells about the shell and Azimuthal Quantum Number tells us about the shape of orbital.
For any orbital,
n=1,2,3,4,...
And l=0 to(n-1)
For example:
n=1 , l= 0
n=2 , l= 0,1
n=3 ,l=0,1,2
(Here 0 stands for s-orbital ,1for p-orbital, 2 for d-orbital and so on..)
So the n(Principal Quantum Number) and l(Azimuthal Quantum Number) values for the following orbitals are:
a)1s:
n=1 (the 1 from "1s")and
l=0 (as 0 represents s-orbital)
b) 3s:
n=3 (the 3 from "3s")and
l =0 (as 0 represents s-orbital)
c)2p:
n=2 (the 2 from "2p")and
l =1 (as 1 represents p-orbital)
d)4d:
n=4 (the 4 from "4d")and
l =2 (as 2 represents d-orbital)
e)5f:
n=5 (the 5 from "5f")and
l =3 (as 3 represents f-orbital)
To learn more about Quantum Numbers :
https://brainly.com/question/14307071
https://brainly.com/question/14694282
The mass ratio of sodium to fluorine in sodium fluoride is 1.21:1. A sample of sodium fluoride produced 23.5 g of sodium upon decomposition. How much fluorine was formed?
Answers
19.42g of fluorine is produced upon decomposition of sodium fluoride.
What is mass ratio?The mass of a given substance is converted to moles using the molar mass of this substance in the periodic table. Moles of a given substance are then converted to moles of an unknown substance using the molar ratios from the balanced chemical formulas.
Mass ratio is defined as the percentage composition of the masses of elements in a molecule or compound. A compound always has a defined mass fraction of the corresponding element.
Mass ratio of sodium to fluorine = 1.21:1
If the mass of sodium fluoride produced is 23.5 g
Using dimensional Analysis,
(23.5g of sodium/sodium fluoride)×(1 g of Fluorine/1.21 g of sodium)
= 19.42g(g of fluorine/g of sodium fluoride)
Mass of fluorine produced = 19.42g
To know more about mass ratio, visit:
https://brainly.com/question/14561456
#SPJ1
A quantity of 1.922 g of methanol (CH3OH) was burned in a constant-volume bomb calorimeter. Consequently, the temperature of the water rose by 4.20 Ce … lsius. If the heat capacity of the bomb plus water was 10.4 kJ/degree Celsius, calculate the molar heat of combustion of methanol
Answers
The formula for calculating the amount of energy or heat released is:
ΔH = C ΔT
where ΔH is heat of combustion, C is heat capacity, while ΔT is change in temperature
ΔH = 8.69 kJ / °C * (5.14°C)
ΔH = 44.67 kJ
Then we calculate the moles of CH3OH which has molar mass of 32.04 g/mol:
moles = 1.922 / 32.04 = 0.05999 mol
SO the molar heat of combustion is:
ΔHm = 44.67 kJ / 0.0599875 mol
ΔHm = 744.60 kJ / mol
1) write a balanced equation to show the hydrolysis of glycerol tristearate (tristearin, a triple ester of glycerol) using water and sodium hydroxide to make soap. in addition to the molar ratios, give the mass ratios, i.e., the ratios of masses that react according to the reaction stoichiometry) based on 20 g of tristearin.
Answers
Mass ratio of Tristearin that react according to the reaction : \(NaOH\) is
20 : 27
The main fat in beef is tristearin. A molecule of glycerine that has interacted with three (3) molecules of the fatty acid stearic acid is known as a triglyceride.
1 mole of Tristearin requires 3 moles of \(NaOH\) to react to give 1 mole of glycerol & 3 moles of sodium sterate.
Molar ratio of reactant Tristearin : \(NaOH\) = 1 : 3
Molar weight of Tristearin = 891.5 g/mol
=> 20 g Tristearin = \(\frac{20}{891.5} moles\)
=> \(NaOH\) required to react with \(\frac{20}{891.5}\) moles of Tristearin = \(3\times\frac{20}{891.5} moles\) = 0.0673 moles
=> Molecular weight of \(NaOH\) = 40 g/mol
=> \(NaOH\) mass required to react with 20 g Tristearin = 0.0673 × 40g = 2.7g
∴ Mass ratio of Tristearin: \(NaOH\) is 20 : 27.
Learn more about Tristearin:
brainly.com/question/7175814
#SPJ4
which element has the electrons configuration 1s22s22p63s23p64s23d104p2
Answers
The element with the electron configuration 1s22s22p63s23p64s23d104p2 is Silicon (Si).
Explanation:
The electron configuration of an element describes the arrangement of electrons in its atoms. The numbers and letters in the configuration represent the energy levels (n), sublevels (s, p, d, f), and the number of electrons in each sublevel.
In this case, the electron configuration of the element is:
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p2
Breaking this down, we can see that the element has:
- 2 electrons in the 1s sublevel
- 2 electrons in the 2s sublevel
- 6 electrons in the 2p sublevel
- 2 electrons in the 3s sublevel
- 6 electrons in the 3p sublevel
- 2 electrons in the 4s sublevel
- 10 electrons in the 3d sublevel
- 2 electrons in the 4p sublevel
Based on the number of electrons in the outermost energy level (valence electrons), we can determine that this element is in group 14 of the periodic table. Looking at the periodic table, we can see that the
An empty balloon sits 10 meters away from a golf ball. Jamie wants to increase the
gravitational force between the two objects by filling the balloon with a substance. Which
of the following substances will most likely increase the gravitational force between the
balloon and the golf ball?
An empty balloon sits 10 meters away from a golf ball. Jamie wants to increase the
gravitational force between the two objects by filling the balloon with a substance. Which
of the following substances will most likely increase the gravitational force between the
balloon and the golf ball?
water
cotton
air
lead pieces
Answers
To increase the gravitational force between the balloon and the golf ball, It should be filled with lead pieces. Option D
What should be done?A substance's density, which measures its mass in relation to its volume, determines how much gravitational force it produces.
Lead bits are one of the suggested materials, and they are the one that would most likely boost the gravitational force. The density of lead is much higher than that of the other listed materials.
The high density of lead will result in an increase in the gravitational pull between the balloon and the golf ball if Jamie fills the balloon with lead bits.
Learn more about density:https://brainly.com/question/29775886
#SPJ1
1. Assume that you have following items and any other necessary equipment.
H₂ gas, O₂ gas, de-ionized water, Li metal, Lit ion-containing proper solution of any
concentration, Zn metal, Zn²+ ion-containing proper solution of any concentration.
(A) Suggest every (theoretically) possible Galvanic cells. The answer must include half-cell
reactions, balanced overall reaction, and Eºcell-
(B) Identify the strongest reducing agent and justify your answer.
(C) If one of the systems suggested in (A) consumes 50g of Zn metal for 2h operation, how
much is the current? How many grams of H₂ can be obtained from water using the
charges of this system?

Answers
(A) Possible Galvanic cells:
Li(s) | Li+ solution || H+ solution | H2(g) | Pt(s)
Half-cell reactions: Li(s) → Li+(aq) + e-; 2H+(aq) + 2e- → H2(g)
Overall reaction: 2Li(s) + 2H+(aq) → 2Li+(aq) + H2(g)
Eºcell = -3.04 V
Zn(s) | Zn2+ solution || H+ solution | H2(g) | Pt(s)
Half-cell reactions: Zn(s) → Zn2+(aq) + 2e-; 2H+(aq) + 2e- → H2(g)
Overall reaction: Zn(s) + 2H+(aq) → Zn2+(aq) + H2(g)
Eºcell = -0.76 V
Zn(s) | Zn2+ solution || O2(g) | H2O(l) | Pt(s)
Half-cell reactions: Zn(s) → Zn2+(aq) + 2e-; O2(g) + 4H+(aq) + 4e- → 2H2O(l)
Overall reaction: Zn(s) + O2(g) + 4H+(aq) → Zn2+(aq) + 2H2O(l)
Eºcell = 1.56 V
(B) The most powerful reducing agent is the one with the lowest Eo value, indicating the greatest proclivity to lose electrons and experience reduction. Based on the Galvanic cells, Li has the most negative Eo value (-3.04 V), making it the most powerful reducing substance.
(c). we can use Faraday's laws of electrolysis to calculate the current and the amount of H₂ gas produced:
Calculate the number of electrons transferred:
From the balanced reaction, we see that 2 moles of electrons (4 e⁻) are transferred for each mole of H₂ produced.
The mass of Zn consumed in 2 hours is 50 g, which is equivalent to 50/65.38 = 0.765 moles of Zn (where 65.38 g/mol is the molar mass of Zn).
Therefore, the total number of electrons transferred is 4 x 0.765 = 3.06 moles of electrons.
Calculate the current:
Faraday's first law states that the amount of chemical change in an electrolytic cell is proportional to the amount of electricity that flows through the cell.
The proportionality constant is the Faraday constant, which is equal to 96,485 C/mol e⁻.
Therefore, the total charge (Q) required to transfer 3.06 moles of electrons is:
Q = 3.06 x 96,485 = 295,038 C
The time (t) taken for this charge to flow through the circuit is 2 hours = 7,200 seconds.
Therefore, the current (I) is:
I = Q/t = 295,038/7,200 = 40.97 A
Calculate the amount of H₂ gas produced:
From the balanced reaction, we know that 1 mole of H₂ gas is produced for every 2 moles of electrons transferred.
Therefore, the number of moles of H₂ gas produced is:
0.765 moles of Zn x (1 mole of H₂/2 moles of electrons) = 0.383 moles of H₂ gas
The molar mass of H₂ is 2 g/mol, so the mass of H₂ gas produced is:
0.383 moles of H₂ gas x 2 g/mol = 0.766 g of H₂ gas
Therefore, the Galvanic cell produces 0.766 g of H₂ gas when it consumes 50 g of Zn metal for 2 hours at a current of 40.97 A.
learn more about Galvanic cells here
https://brainly.com/question/15096829
#SPJ9
a. Using the Born-Mayer Equation, calculate the lattice enthalpy for sphalerite
(zinc blende), ZnS. You must look up the appropriate parameters for the equation.
b. Using the Born-Mayer Equation, calculate the lattice enthalpy for wurtzite, ZnS. You must
look up the appropriate parameters for the equation.
c. Which is thermodynamically stable at ambient conditions (25 °C, 1 bar)? Find a reference
with the T and P phase diagram for ZnS. Submit the pdf of the reference with your file . Also,
compare your answer to the standard enthalpies of formation for wurtzite compared to sphalerite.
Answers
ΔLatticeU = ΔLatticeH – pΔVm is the lattice energy of wurtzite. Ionic compounds often have flat surfaces that meet at distinctive angles and are stiff, brittle, crystalline materials.
Remember that when a metal reacts with a nonmetal, often an ionic compound results from the transfer of electrons form the metal (the reductant) towards the nonmetal (the oxidant). Ionic compounds often have flat surfaces that meet at distinctive angles and are stiff, brittle, crystalline materials. They melt at rather high temperatures and are not easily distorted. ΔLatticeU = ΔLatticeH – pΔVm is the lattice energy of wurtzite.
To know more about lattice energy, here:
https://brainly.com/question/29735933
#SPJ1
When fluids are subjected to increases in pressure they tend to do this.
A.) evaporate
B.) contract
C.) expand
D.) solidify
Answers
Answer:
D.) solidify
Explanation:
7.0×107 ÷ 2.0×104
turn into a proper scientific notation. PLS HELP
Answers
The expression 7.0x\(10^7\) ÷ 2.0x\(10^4\) can be expressed in proper scientific notation as 3.5x10^3.
To express the division 7.0x\(10^7\) ÷ 2.0x\(10^4\) in proper scientific notation, we need to perform the division and adjust the result to the appropriate format.
Dividing the numbers, we get:
7.0x\(10^7\) ÷ 2.0x\(10^4\)= 3.5x\(10^{(7-4)\)= 3.5x\(10^3\)
The result of the division is 3.5, and we adjust the exponent by subtracting the exponent of the divisor from the exponent of the dividend (7 - 4 = 3).
Therefore, the proper scientific notation representation of the division 7.0x\(10^7\) ÷ 2.0x\(10^4\) is 3.5x\(10^3\).
Scientific notation is a way to express numbers using a coefficient (in this case, 3.5) multiplied by a power of 10 (in this case, 10^3). It allows for more concise representation of very large or very small numbers.
In this case, the division resulted in a number that is smaller than the dividend and has a positive exponent, indicating a smaller magnitude compared to the original numbers. The coefficient represents the significant digits of the result, while the power of 10 represents the scale or magnitude of the number.
For more such questions on scientific notation visit:
https://brainly.com/question/28468914
#SPJ8
Methane is burned with oxygen to yield carbon dioxide and water. The feed
contains 20 mole % CH4, 60% 02 and 20% C02 and the conversion rate of the
limiting reactant is 90%.
CHA+2 02--->C02+2H20
Find the following;
1 The limiting reactant.
2- The molar composition of the product stream.
Answers
The limiting reactant of the reaction is CH₄.
The molar composition of the product stream is:
33.33% CO₂66.67% H₂OWhat is the limiting reactant of the reaction?The limiting reactant is determined using the stoichiometric ratios of methane (CH₄) and oxygen (O₂) in the given feed composition.
The balanced equation is:
CH₄ + 2 O₂ ----> CO₂ + 2 H₂O
From the feed composition, we have:
CH₄: 20 mole %
O₂: 60 mole %
CO₂: 20 mole %
Assuming a total feed of 100 moles.
Moles of CH₄ in the feed = 20% of 100 moles = 20 moles
Moles of O2 in the feed = 60% of 100 moles = 60 moles
Based on the balanced equation, the stoichiometric ratio is 1:2 between CH₄ and O₂.
Since we have 20 moles of CH₄ and 60 moles of O₂, we can see that there is an excess of O₂. Therefore, the limiting reactant is CH₄.
The molar composition of the product stream:
Since CH₄ is the limiting reactant, we will determine the amount of carbon dioxide (CO₂) and water (H₂O) produced based on the 90% conversion rate of CH₄.
From the balanced equation, for every mole of CH₄ reacted, we obtain one mole of CO2 and two moles of H₂O.
Moles of CO₂ produced = 90% of 20 moles = 0.9 * 20 = 18 moles
Moles of H₂O produced = 2 * (90% of 20 moles) = 2 * (0.9 * 20) = 36 moles
Total moles of products = Moles of CO2 + Moles of H2O
Total moles of products = 18 moles + 36 moles
Total moles of products = 54 moles
Therefore;
Moles of CO₂ in the product stream = 18 moles / 54 moles * 100% = 33.33%
Moles of H₂O in the product stream = 36 moles / 54 moles * 100% = 66.67%
Learn more about limiting reactants at: https://brainly.com/question/30879855
#SPJ1
Does the NaOH test for calcium ions work when NaOH is added to limewater?
Answers
Answer:
Calcium hydroxide is a white precipitate, too, and it won't dissolve if more sodium hydroxide is added to the solution. No significant precipitate will be formed between calcium ions and ammonia.
Given that a 15.00 g milk chocolate bar contains 9.500 g of sugar, calculate the percentage of sugar present
in 15.00 g of milk chocolate bar keeping in mind that the answer should have four significant figures (two
decimal places).
Answers
Answer:
63.33%
Explanation:
To find the percentage of sugar in the milk chocolate just divide 15 by 9.5. This will give you 0.63333 (recurring). To find the percentage, times it by 100, which will give you 63.33%.
Hope this helped :)
The percentage of sugar present in the milk would be 63.33 %.
What are significant figures?In positional notation, significant figures refer to the digits in a number that is trustworthy and required to denote the amount of something, also known as the significant digits, precision, or resolution.
As given in the problem a 15.00 g milk chocolate bar contains 9.500 grams of sugar, and we have calculated the percentage of sugar present,
The percentage of sugar present in the milk = 9.500 ×100/15
= 63.33 %
Thus, the percentage of sugar present in the milk would be 63.33 %.
To learn more about significant figures here, refer to the link;
brainly.com/question/14359464
#SPJ2
The two most common isotopes of argon (Ar) are argon-36 and argon-40. Given that the average atomic mass is 39.95, what can be said about the relative abundance of each isotope? Which is more common and how do you know? No calculation is necessary. I am looking for a simple written response.
Answers
Answer:
Argon-40
the reason this is abundent is because this is a more common isotope
Explanation:
A 4 M acetic acid (CH₂COOH) solution lowers thefreezing point by -8°C; a 4 M KF solution yields a-15°C freezing-point depression. What can account forthis difference?
Answers
As the freezing point depression equation states,
ΔTf = kf.m.i ,
where kf depends on the solvent, m depends on the concentration and i depends on the particles, the only parameter that changes between the two scenarios presented is i, since it relates to the amount of particles resulted in the dissolution of the solute. While a covalent compound, such as the acetic acid, remains as one particle when it dissolves (i = 1), ionic compounds such as KF dissolves into more particles. Therefore, the i for KF is greater than 1, resulting in a bigger change in freezing temperature.
-Convert 6.02 x 1020 formula units of MgCl₂ to mol of MgCl₂:
Answers
6.02 x \(10^{20\) formula units of MgCl₂ is equal to 0.1 moles of MgCl₂.
To convert formula units of MgCl₂ to moles of MgCl₂, we need to use Avogadro's number, which relates the number of formula units to the number of moles.
Avogadro's number (NA) is approximately 6.022 x 10^23 formula units per mole.
Given that we have 6.02 x 10^20 formula units of MgCl₂, we can set up a conversion factor to convert to moles:
(6.02 x 10^20 formula units MgCl₂) * (1 mol MgCl₂ / (6.022 x 10^23 formula units MgCl₂))
The formula units of MgCl₂ cancel out, and we are left with moles of MgCl₂:
(6.02 x 10^20) * (1 mol / 6.022 x 10^23) = 0.1 mol
Therefore, 6.02 x 10^20 formula units of MgCl₂ is equal to 0.1 moles of MgCl₂.
It's important to note that this conversion assumes that each formula unit of MgCl₂ represents one mole of MgCl₂. This is based on the stoichiometry of the compound, where there is one mole of MgCl₂ for every one formula unit.
Additionally, this conversion is valid for any substance, not just MgCl₂, as long as you know the value of Avogadro's number and the number of formula units or particles you have.
For more such questions on MgCl₂ visit:
https://brainly.com/question/26311875
#SPJ8
Why does our body need to use both mechanical and chemical digestion when we are breaking down our food?
Answers
Answer:
Chemical digestion is a vital part of the digestive process. Without it, your body wouldn't be able to absorb nutrients from the foods you eat, also chemical digestion uses enzymes to break down food
mechanical digestion involves physical movements, such as chewing and muscle contractions,
Explanation:
738.90 m has ____ significant figures
Answers
Answer: 4
Explanation: because the zero doesn't count
30 points!! please help chem question

Answers
the state of y at room temperature is gas, good luck!
How do cows contribute to the production of greenhouse gases?
Answers
Answer:
Cattle produce a lot of methane gas, primarily through enteric fermentation and fermentation of their manure. Methane is a powerful greenhouse gas that, along with nitrous oxide, carbon dioxide, and some other compounds in the atmosphere, create a blanket around our planet.
Answer:
They add methane gas through enteric fermentation and fermentation of their manure
Explanation:
13. Gobar gas mainly contains. a) Ethane b) Methane c) Propane d) Butane
Answers
Hello !
Gobar gas mainly contains methane.
It is mainly a mixture of mainly methane and carbon dioxide. Methane is a major component of biogas.
An intravenous solution is noted as having a concentration of glucose equal to 0.312 M. What volume of solution (in mL) is needed to deliver 0.078moles of glucose to the patient?Select one:a. 312 mLb. 4 mLc. 0.4mLd 1000 mLe. 250 mL
Answers
INFORMATION:
We know that:
- An intravenous solution has a concentration of glucose equal to 0.312 M
And we must find the volume of solution needed to deliver 0.078 moles of glucose to the patient
STEP BY STEP EXPLANATION:
To find it, we need to use the formula of Molarity (concentration)
\(Molarity=\frac{moles\text{ of solute}}{Liters\text{ of solution}}\)Is given that,
- Molarity = 0.312 M = 0.312 mol/L
- moles of solute = 0.078 mol
Now, replacing in the formula
\(0.312\frac{mol}{L}=\frac{0.078mol}{Liters\text{ of solution}}\)Then, solving for liters of solution
\(Liters\text{ of solution}=\frac{0.078mol}{0.312\frac{mol}{L}}=0.25L\)Finally, since we need the volu
Determine the volume (L) of nitrogen monoxide gas that is created at STP when 32.2 g
of solid copper reacts with excess nitric acid.
3Cu(s) + 8HNO3(aq) — 3Cu(NO3)2 (aq) + 4H2O(1) + 2NO(g)
Answers
Taking into account the reaction stoichiometry and STP conditions, the volume of nitrogen monoxide gas that is created at STP when 32.2 g of solid copper reacts with excess nitric acid is 7.5677 L.
Reaction stoichiometryThe balanced reaction is:
3 Cu(s) + 8 HNO₃(aq) → 3 Cu(NO₃)₂ (aq) + 4 H₂O(l) + 2 NO(g)
By reaction stoichiometry, the following amounts of moles of each compound participate in the reaction:
Cu: 3 molesHNO₃: 8 molesCu(NO₃)₂: 3 molesH₂O: 4 moles NO: 2 molesThe molar mass of the compounds is:
Cu: 63.54 g/moleHNO₃: 63 g/moleCu(NO₃)₂: 187.54 g/moleH₂O: 18 g/moleNO: 30 g/moleBy reaction stoichiometry, the following mass quantities of each compound participate in the reaction:
Cu: 3 moles× 63.54 g/mole= 190.62 gramsHNO₃: 8 moles× 63 g/mole= 504 gramsCu(NO₃)₂: 3 moles ×187.54 g/mole= 562.62 gramsH₂O: 4 moles ×18 g/mole= 72 gramsNO: 2 moles ×30 g/mole= 60 gramsSTP conditionsThe STP conditions refer to the standard temperature and pressure, which values are 0 °C and 1 atmosphere and are reference values for gases. In these conditions 1 mole of any gas occupies an approximate volume of 22.4 liters.
Moles of NO formedThe following rule of three can be applied: if by reaction stoichiometry 190.62 grams of Cu form 2 moles of NO, 32.2 grams of Cu form how many moles of NO?
moles of NO= (32.2 grams of Cu× 2 moles of NO)÷ 190.62 grams of Cu
moles of NO= 0.3378 moles
Then, 0.3378 moles of NO are formed.
Volume of NO createdNow, you can apply the following rule of three: if by definition of STP conditions 1 mole of NO occupies a volume of 22.4 liters, 0.3378 moles occupies how much volume?
volume= (0.3378 moles× 22.4 L)÷ 1 mole
volume= 7.5677 L
Finally, the volume of NO created is 7.5677 L.
Learn more about the reaction stoichiometry:
brainly.com/question/24741074
#SPJ1
Net ionic equation for potassium sulfide and magnesium iodide
Answers
The net ionic equation for the reaction between potassium sulfide and magnesium iodide is S2- + Mg2+ -> MgS, as the potassium and iodide ions are spectator ions and do not participate in the reaction.
To determine the net ionic equation for the reaction between potassium sulfide (K2S) and magnesium iodide (MgI2), we first need to identify the ions present in each compound and then determine the products formed when they react.
Potassium sulfide (K2S) dissociates into two potassium ions (K+) and one sulfide ion (S2-):
K2S -> 2K+ + S2-
Magnesium iodide (MgI2) dissociates into one magnesium ion (Mg2+) and two iodide ions (I-):
MgI2 -> Mg2+ + 2I-
Now, we need to determine the possible products when these ions combine. Since potassium (K+) has a +1 charge and iodide (I-) has a -1 charge, they can combine to form potassium iodide (KI):
K+ + I- -> KI
Similarly, magnesium (Mg2+) and sulfide (S2-) can combine to form magnesium sulfide (MgS):
Mg2+ + S2- -> MgS
Now, we can write the complete ionic equation by representing all the ions present before and after the reaction:
2K+ + S2- + Mg2+ + 2I- -> 2KI + MgS
To obtain the net ionic equation, we remove the spectator ions, which are the ions that appear on both sides of the equation and do not participate in the actual reaction. In this case, the spectator ions are the potassium ions (K+) and the iodide ions (I-).
Thus, the net ionic equation for the reaction between potassium sulfide and magnesium iodide is:
S2- + Mg2+ -> MgS
For more such questions on ionic equation visit:
https://brainly.com/question/25604204
#SPJ8
a. Phenol (C6H5OH) is an aromatic compound and a colourless liquid widely used in household products
and medicine. When it reacts with chlorine liquid (chlorine is a diatomic molecule), in the presence of a
catalyst, one of the aromatic hydrogen atoms attached directly to a C atom is replaced with a chlorine
atom, and liquid chlorophenol is formed. This replacement process is called chlorination
Write a balanced chemical equation for the chlorination reaction and explain how you balanced it. Note
that hydrogen chloride gas (HCI) is also a product of this chemical reaction and you should ignore the
presence of the catalyst in the equation.
Answers
Because there are 2 Cl on the left, we will put a coefficient 2in front of HCl on the right side to balance out the Cl. This would result in an unequal amount of H, with 6 on the right side and 7 in the left, so we have to put a coefficient of 2 in front of C6H5OH and C6H4OH on both sides to balance out the H. By doing this, we would obtain an equal amount of H on both sides. The Carbon is already balanced, and so is the Oxygen.
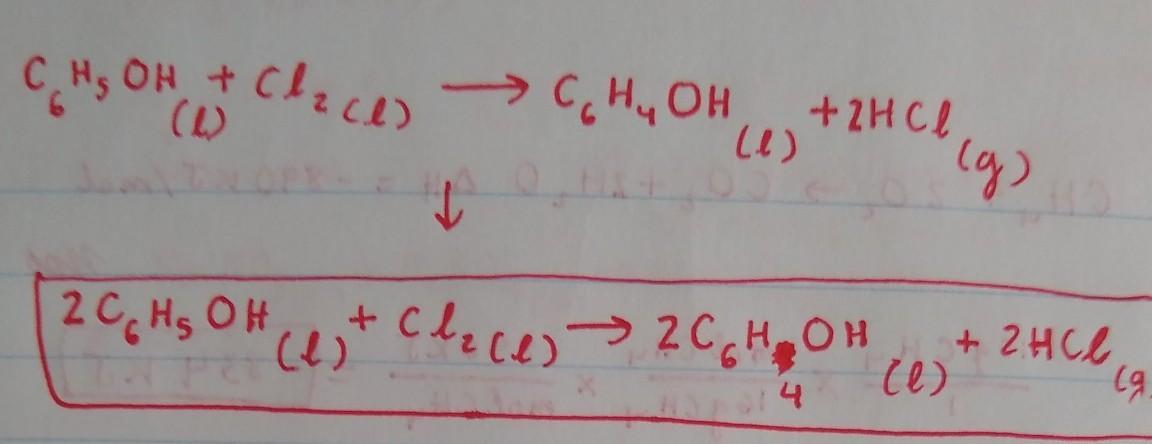
The balanced chemical reaction equation for the reaction between aromatic phenol and chlorine gas in the presence of FeCl3 as catalyst is as follows;
C6H6O + Cl2 -------> C6H5OCl + HCl
An aromatic compound has 4n + 2 number of pi electrons. This condition is satisfied by phenol. Hence, phenol has the stability associated with aromatic compounds.
The reaction of phenol with chlorine in the presence of a catalyst such as FeCl3 is an aromatic electrophillic substitution reaction.
This reaction yields a chlorinated phenol (one of the aromatic hydrogen atoms attached directly to a C atom is replaced with a chlorine atom and chlorophenol is formed).
A balanced chemical reaction equation is one in which the number of atoms of each element at the reactant side and the product side are equal. This condition is satisfied for the reaction;
C6H6O + Cl2 -------> C6H5OCl + HCl
Learn more; https://brainly.com/question/6170291
Need your help ASAP, please!
What is the molarity of a 4.9% H2SO4 solution of density 0.98 gm/ml?
The answer is 0.49 M, please do explain how
Answers
0.49 M is the molarity of the 4.9% \(H_{2}SO_{4}\) solution.
To find the molarity of a solution, we need to calculate the number of moles of the solute (\(H_{2}SO_{4}\)) present in a given volume of the solution. Here's how to determine the molarity of a 4.9% \(H_{2}SO_{4}\)solution with a density of 0.98 g/ml:
Determine the mass of the solute: The 4.9% concentration implies that 4.9 g of \(H_{2}SO_{4}\) is present in 100 g of the solution.
Calculate the volume of the solution: Divide the mass of the solution by its density. In this case, 100 g / 0.98 g/ml = 102.04 ml.
Convert the volume of the solution to liters: Divide the volume by 1000 to convert from milliliters to liters. 102.04 ml / 1000 = 0.10204 L.
Calculate the number of moles: Multiply the mass of the solute by its molar mass. The molar mass of \(H_{2}SO_{4}\) is 98.09 g/mol. Therefore, 4.9 g / 98.09 g/mol = 0.0499 mol.
Calculate the molarity: Divide the number of moles by the volume of the solution in liters. 0.0499 mol / 0.10204 L ≈ 0.49 M.
Thus, the molarity of the 4.9% \(H_{2}SO_{4}\) solution is approximately 0.49 M.
Know more about molarity here:
https://brainly.com/question/30404105
#SPJ8
predict the direction favored of the following equilibrium equation

Answers
Comparing the stabilities of two negative charges on opposite sides of the equilibrium-arrows is one method for determining whether the reactants or products are preferred in an equilibrium. Because this side has less energy, it is preferred, whichever side has the more stable negative charge.
How do you know which side is favored at equilibrium?It is well knowledge that nature prefers low energy conditions. Remember that we may use the Protocol to compare the stability of two negative charges, where the more stable negative charge has a lower energy, and that this evaluation can be done while keeping this concept in mind.Comparing the stabilities of two negative charges on opposite sides of the equilibrium-arrows is one method for determining whether the reactants or products are preferred in an equilibrium. Because this side has less energy, it is preferred, whichever side has the more stable negative charge.Considering what transpires once you reach the more stable negative charge is one method to understand why this assertion is correct.Because you are content with where you are—a place of low energy—if you have recently moved to a more solid job, you are less likely to react negatively.By contrasting the negative charges on an oxygen and a nitrogen in the aforementioned example, we are putting the protocol to use. Since the charges on two atoms in the same row of the periodic table are being compared, we use electronegativity (#2 on the Protocol) to ascertain the relative stabilities.To Learn more About equilibrium refer To:
https://brainly.com/question/26075805
#SPJ1

How many atoms (or molecules) are present in 1 mole?Question 19 options:A) 1B) 6.022×1020C) 6.022×1023D) 6.022
Answers
Answer:
The answer is C.
Explanation:
The number of particles (atoms/molecules) in 1 mole is referred to as the Avogadro's number. The Avogadro's number = 6.022 * 10 ^ 23. Therefore, the answer is C.
At STP, how many liters of hydrogen are needed to react with 88 g of copper (II) oxide?
Answers
Answer:
So if we need to react with 88 gm. of copper 2 then at least 3-4 liters.
Explanation:
A 2.538 g sample of iron combines with chlorine gas to form an iron chloride with a mass of 7.25 g. What is the empirical formula of the iron chlorine?
Answers
A 2.538 g sample of iron combines with chlorine gas to form an iron chloride with a mass of 7.25 g.The empirical formula of the iron chlorine is FeCl₅.
What do you mean by empirical formula?The term empirical formula is defined as a chemical formula that exhibits the simplest whole-number ratio of the atoms in a molecule or compound.
It is obtained from experimental data such as the masses of the elements in a compound.
Given:
Number of moles of the iron
= 2.538 g/56 g/mol
= 0.045
Number of moles of chlorine = 0.204 moles
By dividing through by the lowest ratio
we have that the mole ratio is; 1: 5
Thus, the empirical formula of the iron chlorine is FeCl₅
Learn more about empirical formula:
brainly.com/question/14044066
#SPJ1