Answers
Answer:
\(T_2 =388.50K\)
Explanation:
Given
\(p_1 = ??\) -- Initial vapour pressure
\(p_2 = 6.50p_1\) --- Final vapour pressure
\(T_1 = 355K\) ---- Initial temperature
\(T_2 = ??\) --- Final temperature
\(\triangle _{vap}H = 64.08kJmol\) --- Enthalpy of vaporization
Required
Calculate T2
To do this, we make use of Clausius-Clapeyron equation.
Which states that:
\(ln(\frac{p_2}{p_1}) = \frac{\triangle _{vap}H}{R}(\frac{1}{T_1} - \frac{1}{T_2})\)
Where:
\(R = 8.314 J.K^{-1}mol^{-1}\) --- Universal Gas constant
\(\triangle _{vap}H = 64.08kJmol\)
\(\triangle _{vap}H = 64080\ kJmol\)
\(ln(\frac{p_2}{p_1}) = \frac{\triangle _{vap}H}{R}(\frac{1}{T_1} - \frac{1}{T_2})\) becomes
\(ln(\frac{6.50p_1}{p_1}) = \frac{64080}{8.314}(\frac{1}{355} - \frac{1}{T_2})\)
\(ln(\frac{6.50p_1}{p_1}) = 7707.48(\frac{1}{355} - \frac{1}{T_2})\)
\(ln(6.50) = 7707.48(\frac{1}{355} - \frac{1}{T_2})\)
\(1.872 = 7707.48(\frac{1}{355} - \frac{1}{T_2})\)
Take LCM
\(1.872 = 7707.48(\frac{T_2 - 355}{355T_2})\)
\(1.872 = 7707.48*\frac{T_2 - 355}{355T_2}\)
\(1.872 = \frac{7707.48*(T_2 - 355)}{355T_2}\)
Cross Multiply
\(355T_2*1.872 = 7707.48*(T_2 - 355)\)
\(664.56T_2 = 7707.48T_2 - 2736155.4\)
Collect Like Terms
\(664.56T_2 - 7707.48T_2 =- 2736155.4\)
\(-7042.92T_2 =- 2736155.4\)
Make T2 the subject
\(T_2 =\frac{- 2736155.4}{-7042.92}\)
\(T_2 =388.497299416\)
\(T_2 =388.50K\)
The final temperature is 388.50K
The temperature at which the vapor pressure will be 6.50 times higher than it was at 355K is 389 K
The Clausius-Clapeyron Equation is widely used for the determination of the vapor pressure of another temperature, provided we know the vapor pressure at a certain temperature as well as the heat of vaporization.
Given that:
the heat of vaporization \(\mathbf{\Delta H_{vap} = 64.08 \ kJ/mol = 64.08 \times 10^3 J/mol}\) the initial temperature T₁ = 355 Kthe final temperature T₂ = ???if the pressure at T₁ = P₁then, the pressure at T₂ = 6.5P₁the universal gas constant = 8.314 J/K/molBy using the Clausius-Clayperon Equation:
\(\mathbf{In \Big( \dfrac{P_2}{P_1} \Big) = \dfrac{\Delta H_{vap}}{R} \Big (\dfrac{1}{T_1} - \dfrac{1}{T_2} \Big) }\)
\(\mathbf{In \Big( \dfrac{6.5P_1}{P_1} \Big) = \dfrac{64.08 \times 10^3 }{8.314} \Big (\dfrac{1}{355} - \dfrac{1}{T_2} \Big) }\)
\(\mathbf{In(6.5)=7707.48 \Big (\dfrac{1}{355} - \dfrac{1}{T_2} \Big) }\)
\(\mathbf{1.872 =21.71 - \dfrac{7707.48}{T_2} }\)
\(\mathbf{\dfrac{7707.48}{T_2} = 21.71- 1.872 }\)
\(\mathbf{\dfrac{7707.48}{T_2} = 19.838 }\)
\(\mathbf{T_2= \dfrac{7707.48}{19.838} }\)
\(\mathbf{{T_2}=388.521 \ K }\)
T₂ ≅ 389 K
Learn more about Clausius-Clayperon equation here:
https://brainly.com/question/1077674?referrer=searchResults
Related Questions
Cho một lượng hỗn hợp CuO và Fe2O3 tác dụng hết với dung dịch HCl thu được 2 muối có tỉ lệ mol là 1 : 1. Phần trăm khối lượng của CuO và Fe2O3 trong hỗn hợp lần lượt là:
Answers
Answer:
fafsafwafsxDlmfao
Explanation:
Solve this organic transformation....use - Br2,CCl4,KOH,CH3OH,Hg+2,diluted H2SO4, PCC,HBr,Mg,Dry ether,Na,H2,Pd,quinoline
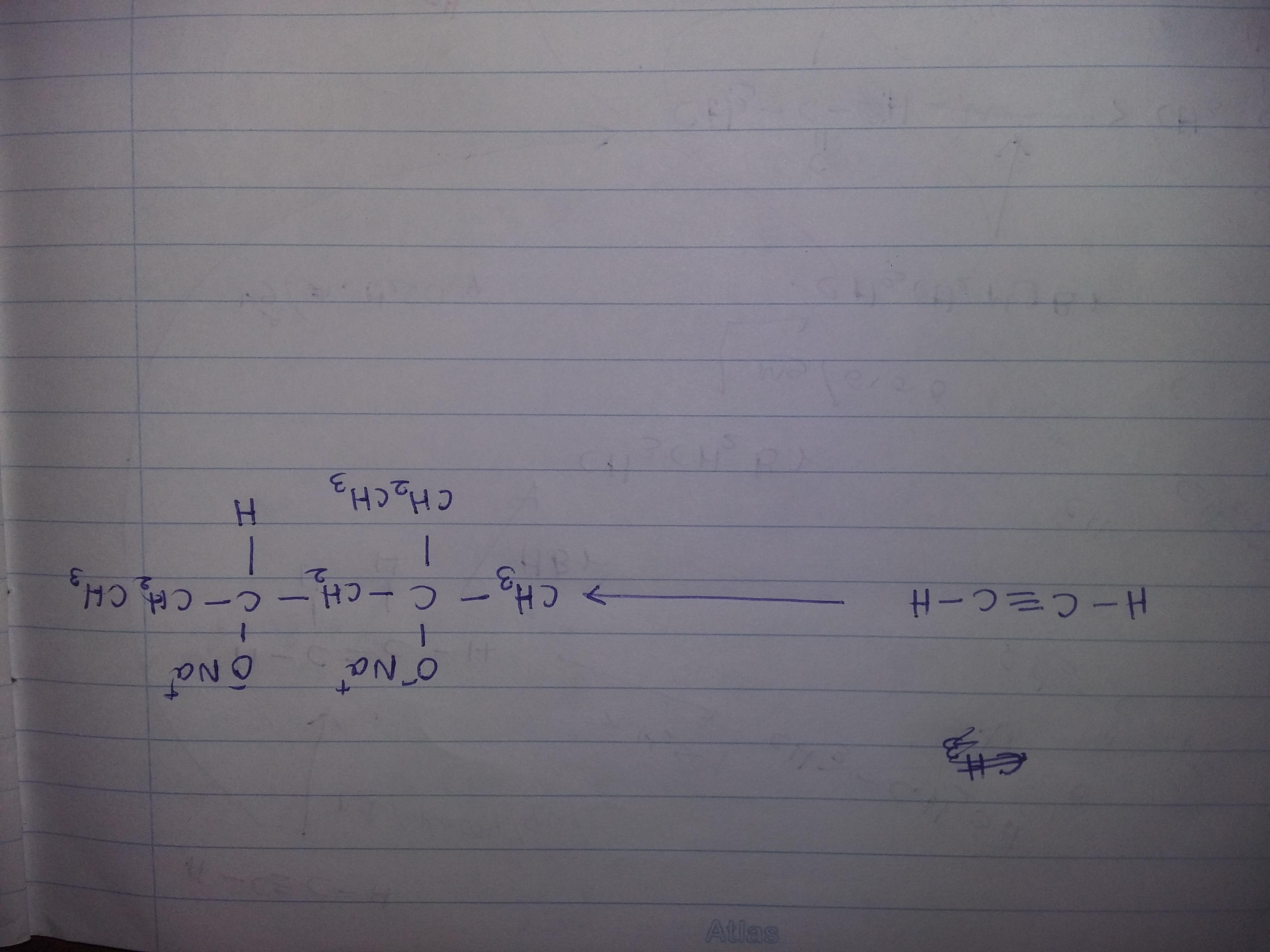
Answers
Organic transformation sequential equation using catalysts will be as follows:
2CH3-CH2-O => (alc. KOH) => CH2=CH2 + KCl + H2O => (Br2/CCl4) => CH2Br-CH2Br + Zn
CH2Br-CH2Br + Zn => (HBr /Pd) => CH2=CH2+ZnBr2
As can be visualized from above organic transformation equation, conversion of dry ether in presence of alkaline potassium hydroxide results in formation of unstable ethene. This dry unsaturated compound of ethene is stabilized by reaction that happens in presence of bromine or calcium tetrachloride as the catalyst which results in formation of ethylene bromide which in presence of highly efficient palladium as catalyst results in formation of stable ethene as byproduct. Thereby with formation of stable compound of ethylene, it releases zinc bromide as byproduct resulting completion of reaction equation. This stable product ethene is a double bonded carbon structure that is chemically extremely flammable and has planar structure.
To know more about organic transformation:
brainly.com/question/14413579
#SPJ1
The force that holds protons together is called...?
Answers
Answer: Answer is in Explanation
Explanation:
The strong nuclear force pulls together protons and neutrons in the nucleus. At very small distances only, such as those inside the nucleus, this strong force overcomes the electromagnetic force, and prevents the electrical repulsion of protons from blowing the nucleus apart.
How many particles are in 3.4 moles of NaCl?
Answers
Answer:
The answer is
2.049 × 10²⁴ particlesExplanation:
To find the number of entities or particles given the number of moles of a substance we use the formula
N = n × Lwhere n is the number of moles
N is the number of entities
L is the Avogadro's constant which is
6.02 × 10²³ entities
From the question
n = 3.4 mol
We have
N = 3.4 × 6.02 × 10²³
We have the final answer as
2.049 × 10²⁴ particlesHope this helps you
Which metal ion is responsible for the red firework ?
Answers
Answer:
I'm pretty sure that's Strontium
Strontium ion is responsible for the red firework.
Strontium is the chemical element with the image Sr and atomic variety 38. An alkaline earth steel, strontium is a gentle silver-white yellowish steel detail this is fantastically chemically reactive.
What is a metal ion?A metal ion is a type of atomic compound that has an electrical charge. Such atoms willingly lose electrons to form positive ions called cations. Ions are essentially capped by delocalized electrons, which are responsible for processes such as conductivity.
What is a firework?Fireworks are a class of low-explosion fireworks equipment used for aesthetic and recreational purposes. They are most commonly used at fireworks festivals (also known as fireworks shows or fireworks) and combine numerous devices in outdoor settings. Such exhibitions are the focus of many cultural and religious celebrations.
Learn more about metal ion here https://brainly.com/question/11185179
#SPJ2
How can you use density to separate mixtures like sand and small plastic pellets?
Answers
Answer:
Slide a magnet through the mixture. When you take the magnet off, the iron filings are going to be attracted to the magnet and the sand will stay in the bowl
Explanation:
Answer:
Density can be used to separate the substances that make up a mixture, because each substance in a mixture has its own density. For example, if a mixture of sand and oil is placed in water, the sand will sink to the bottom of the container. The sand is more dense than the water.
Explanation:
Please mark me as brainy if this helps
An actual measurement of a sample 3.5 M the measured value is 2.5 M what is the percentage error in the measurement
Answers
Answer: 29%
Explanation:
Answer:
b 29 percent
Explanation:
reaction will be spontaneous at all temperatures if _____
Answers
If a reaction has a negative ΔG and a positive ΔS, the reaction will be spontaneous at all temperatures.
If a reaction is spontaneous at all temperatures, it implies that the reaction will occur without the need for any external intervention, such as the addition of energy. For a reaction to be spontaneous, it must satisfy the criteria of thermodynamic favorability, which is determined by the change in Gibbs free energy (ΔG) associated with the reaction.
The relationship between ΔG, temperature (T), and the equilibrium constant (K) of a reaction is described by the equation ΔG = ΔH - TΔS, where ΔH is the change in enthalpy and ΔS is the change in entropy.
To ensure spontaneity at all temperatures, two conditions must be met:
ΔG must be negative: A negative ΔG indicates a thermodynamically favorable reaction, meaning the products have a lower Gibbs free energy than the reactants. If ΔG is negative, the reaction will proceed spontaneously in the forward direction.
ΔS must be positive: A positive ΔS signifies an increase in the overall entropy of the system. Higher entropy means more disorder, and spontaneous reactions often involve an increase in randomness. When ΔS is positive, it can compensate for the enthalpic term, ΔH, allowing the reaction to proceed spontaneously.
For more such questions on spontaneous visit:
https://brainly.com/question/30127476
#SPJ8
In a chemical reaction
Zn(NO3)2 + NO2 + H₂O
a) What is redox reaction?.
b) Balance the reaction by oxidation number or ion electron method.
Answers
A redox reaction is a reaction in oxidation or the loss of electrons occurs simultaneously with reduction involving a gain of electrons.
The balanced equation of the redox reaction by the oxidation number method is as follows: Zn + 2HNO₃ ----> Zn(NO₃)₂ + 2NO₂ + H₂O
What is the balanced equation of the redox reaction?The complete equation of the redox reaction is given below as follows:
Zn + HNO₃ ----> Zn(NO₃)₂ + NO₂ + H₂OTo balance the chemical reaction by oxidation number, we need to ensure that the total change in oxidation numbers for each element is zero on both sides of the equation.
Let's assign the oxidation numbers to the elements:
In Zn(NO₃)₂, the oxidation number of Zn is +2, and the oxidation number of each NO₃ group is -1.
In HNO₃, the oxidation number of H is +1, the oxidation number of N is +5, and the oxidation number of each O in NO₃ is -2.
On the product side, the oxidation number of Zn is +2, and the oxidation number of each NO₃ group is -1. The oxidation number of N in NO₂ is +4, and the oxidation number of each O is -2. The oxidation number of H in H₂O is +1, and the oxidation number of O is -2.
Now, let's balance the reaction by considering the changes in oxidation numbers:
Zn: 0 → +2
H: +1 → 0
N: +5 → +4
O: -2 → -2
To balance the oxidation numbers, we need two NO₂ molecules on the product side. The balanced equation is:
Zn + 2HNO₃ ----> Zn(NO₃)₂ + 2NO₂ + H₂O
Learn more about redox reactions at: https://brainly.com/question/21851295
#SPJ1
Mg(s) + Ni2+(ag) -> Mg2+ (aq) + Ni(s) What is the total number of moles of electrons lost by Mg(s) when 2.0 moles of electrons are gained by Ni2+(ag)? * 10 ( 1.0 mol ,20 mol ,3.0 mol, 4.0 mol
Answers
The total number of moles of electrons lost by Mg(s) when 2.0 moles of electrons are gained by Ni2+(ag) is also 2.0 moles of electrons.
How to find the number of moles?This is because in a chemical equation, the number of moles of electrons gained by the reducing agent (in this case Ni2+) is equal to the number of moles of electrons lost by the oxidizing agent (in this case Mg(s)).
In this redox reaction, Mg is being oxidized because it loses electrons and Ni is being reduced because it gains electrons. The oxidation and reduction process are occurring simultaneously, so the number of electrons lost by Mg(s) is equal to the number of electrons gained by Ni2+(ag).
Learn more about moles of electrons in brainly.com/question/512038
#SPJ1
The electrons that are gained by the \(Ni^{2+}\) ion is 2.0 moles of electrons.
What is the number of the electrons gained?We know that when there is a redox reaction, there would be the loss or gain of electrons in the process. The process is a simultaneous one so the electrons that are lost by one specie must as a matter of necessity be gained by another specie.
In this case, as we look at the reaction equation we can see that there are two electrons that have been lost by the magnesium atom and these two electrons would be gained by the Nickel II ion.
Learn more about redox reaction:https://brainly.com/question/13293425
#SPJ1
Directions for a lab require heating a reactant to a temperature of 363.6 K. However, the only thermometer available reads in degrees Celsius. What is this temperature in °C?
Answers
Answer:The suggested answers are for K=298 degrees and the nearest correct answer seems to be increase the room temperature by 22 degrees Fahrenheit. But by calculation, for 300 K, then convert 300k to degrees Celsius = 300-273.15=26.85 degrees celsius. Then convert the 26.85 to degrees F, so F=9/5C + 32= 48.33+32=80.33-55F (present room temperature)=25.33 degrees F to increase the room temperature by.
Explanation:
According to conversion between Celsius and kelvin temperatures , 363.6 K is 90.6°C.
What is the conversion between kelvin and Celsius scales?Three scales are used to measure temperature the Kelvin scale, Celsius scale and Fahrenheit scale.The basic unit of temperature which is used in SI system is kelvin temperature however it is impractical in most practical uses.
The Celsius scale is commonly used in clinical terms where it is related to kelvin scale as the two units are equal in magnitude. The difference between Celsius and kelvin scale is the zero point which on kelvin scale represents theoretical temperature at which substance has no further heat loss.
While the zero point on Celsius scale represents freezing point of water. The two scales are related as follows
K=°C+273
Learn more about conversion between Celsius and kelvin,here:
https://brainly.com/question/3168451
#SPJ2
As sugar water solution made from 110 grams of sugar( gluecose, molar mass= 180.16 g/mol) and 500 grams of water. What is the molality of this solution?
Answers
To solve this question, we have to convert the grams of glucose to moles using its molecular weigth and convert the grams of water to kilograms of water using a conversion factor:
\(110gGlucose\cdot\frac{1molGlucose}{180.16gGlucose}=0.61molGlucose\)\(500gH_2O\cdot\frac{1kg}{1000g}=0.5kgH_2O\)Now, divide the number of moles of glucose by the number of kg of water to find the molality of the solution:
\(m=\frac{0.61mol}{0.5kg}=1.22m\)5. A company uses a substance that is a solid under normal conditions. This substance will be used in extreme conditions, which could make the substance’s molecules move faster and cause a phase change. How would this phase change occur, and how would the molecules of the substance be affected under these extreme conditions?
Energy would be transferred . . .
Answers
Answer:
The shift often happens when heat is applied or removed at a specific temperature
Explanation:
PLEASE MARK ME BRANLY TY HAVE A GREAT DAY!
Does 4 have an infinate number of significant digits?
Answers
Please Help ASAP!!
100 pts + Brainliest!!

Answers
Answer:
Hope this helps ;) don't forget to rate this answer !
Explanation:
The molar mass of a sample can be calculated using the formula:
Molar mass = (molecular weight) / (number of moles)
To find the molar mass, we first need to find the molecular weight of the sample. The molecular weight is the weight of a single molecule of the substance in atomic mass units (amu).
To find the molecular weight, we need to convert the weight of the molecule from grams to amu. One amu is equal to 1.66 x 10^-24 grams.
So, to convert the weight of the molecule from grams to amu, we can divide the weight of the molecule (5.34 x 10^-23 grams) by the conversion factor (1.66 x 10^-24 grams/amu):
Molecular weight (amu) = (5.34 x 10^-23 grams) / (1.66 x 10^-24 grams/amu)
= 3.22 x 10^-22 amu
Now that we have the molecular weight, we can use it to calculate the molar mass. However, we need to know the number of moles in order to do this. Without this information, it is not possible to calculate the molar mass.
Answer:
i agree
Explanation:
How many grams of Br2 are needed to form 67.1 g of AlBr3 ?
2Al(s)+3Br2(l)⟶2AlBr3(s)
Answers
Answer:
Approximately \(60.3\; \rm g\).
Explanation:
Look up the relative atomic mass of \(\rm Al\) and \(\rm Br\) on a modern periodic table:
\(\rm Al\): \(26.982\).\(\rm Br\): \(79.904\).Calculate the formula mass of \(\rm AlBr_3\) and \(\rm Br_2\):
\(\begin{aligned}& M(\mathrm{AlBr_3}) = 26.982 + 3 \times 79.904 \approx 266.694\; \rm g \cdot mol^{-1} \\ & M(\mathrm{Br_2}) = 2\times 79.904 \approx 159.808\; \rm g \cdot mol^{-1}\end{aligned}\).
Calculate the number of moles of formula units in \(67.1\; \rm g\) of \(\rm AlBr_3\):
\(\begin{aligned}n(\mathrm{AlBr_3}) &= \frac{m(\mathrm{AlBr_3})}{M(\mathrm{AlBr_3})} \\ &\approx \frac{67.1\; \rm g}{266.694\; \rm g \cdot mol^{-1}} \approx 0.2516\; \rm mol \end{aligned}\).
Refer to the balanced equation for this reaction. The ratio between the coefficients of \(\rm Br_2\) and \(\rm AlBr_3\) in that equation is three-to-two. That corresponds to the ratio:
\(\begin{aligned}\frac{n(\text{$\mathrm{Br_2}$, consumed})}{n(\text{$\mathrm{AlBr_3}$, produced})} &= \frac{3}{2}\end{aligned}\).
It is already calculated that approximately \(0.2516\; \rm mol\) of \(\rm AlBr_3\) was produced through this reaction. Apply this ratio to approximate the (minimum) number of moles of \(\rm Br_2\) that is consumed:
\(\displaystyle \frac{3}{2} \times 0.2516\; \rm mol \approx 0.3774\; \rm mol\).
Calculate the mass of that \(0.3774\; \rm mol\) of \(\rm Br_2\):
\(\begin{aligned}m(\mathrm{Br_2}) &= n(\mathrm{Br_2})\cdot M(\mathrm{Br_2}) \\ &\approx 0.3774\; \rm mol \times 159.808\; \rm g \cdot mol^{-1} \approx 60.3\; \rm g\end{aligned}\).
Heart, 5 stars, and Brainiest to first right answer!
Which two types of air masses would likely form a subtropical jet stream?
Warm and cool air masses meeting near the North Pole
Cool and warm air masses meeting near the equator
Multiple warm air masses meeting near the equator
Two or more cool air masses meeting near the South Pole
Answers
Answer:
Give person above me brainliest
Explanation:
next is the retrosynthesis of the acyl chloride from tert-butyl bromide. choose the best option for the precursor needed to make the acid chloride. 1949p4 1949p4c 1949p4b 1949p4a 1949p4e 1949p4d
Answers
Option A.The reaction of a carboxylic acid with SOCl2 produces acid chloride as a product.
Acid chlorides have the molecular formula RCOCl, where R is the side chain. They are carboxylic acid reactive derivatives. Acyl chloride is an organic compound consisting of a chlorine atom attached to an acyl group in organic chemistry.
Acyl chlorides are used to make anhydride amides and esters by reacting acid chlorides with salts of carboxylic acids, amines, or alcohols. There are some related compounds in which the -OH group of the acid is replaced by another. Such compounds are called acid derivatives.
Learn more about Acid chlorides here:- https://brainly.com/question/14315479
#SPJ4

PLZ HELP IM GIVING 50 FRICKING POINTS. NO WRONG ANSWERS PLZ

Answers
Answer:
I think it's B
Explanation:
I dont have much experience with the periodic table, but I just think its B.
Answer:
it's either B or C
Explanation:
I don't have that much experience for this but im giving you my honest answer i hope this will help ;3
which statement about the sun is true?
A.The sun is a red giant
B. The sun will become a red giant
C. The sun began as a red giant
D. The sun cannnot become a reg giant
Answers
C H 2 above single bond C H 3 below, C H 2 single bond C H 2 single bond C H above, single bond C H 2 below single bond C H 3 below, single bond above C H 2 single bond C H 3 below

Answers
The first step to name the given alkane is to identify its longest carbon chain, which will be the main chain of the organic molecule:
This chain contains 7 carbons, which means that the compound is a heptane.
Now, we have to identify the branches of the chain:
This chain has 2 carbons, which is named as ethyl, and it is located at the third carbon of the chain, therefore, the full name of this compound is:
3-ethyl-heptane


Suppose that you move from a Suppose that you move from a town near the ocean to a town in the mountains. To what atmospheric changes would your body need to adjust? town near the ocean to a town in the mountains. To what atmospheric changes would your body need to adjust?
Answers
Answer:
all I can say is town near the ocean atmospheric changes will be cooler, warm, sea breeze, and fresh healthy air. Then when it comes to the mountain lot of change firstly there's a dry air
what mass of glucose c6h12o6 would be required to prepare 5000 mL of a 0.215 M solution
Answers
Approximately 194.0 grams of glucose (C6H12O6) would be required to prepare a 5000 mL solution with a concentration of 0.215 M.
To determine the mass of glucose (C6H12O6) required to prepare a 0.215 M solution in 5000 mL, we need to use the formula:
Molarity (M) = moles of solute / volume of solution (in liters)
First, let's convert the volume of the solution from milliliters (mL) to liters (L):
5000 mL = 5000/1000 = 5 L
Now, we can rearrange the formula to solve for moles of solute:
moles of solute = Molarity (M) x volume of solution (L)
moles of solute = 0.215 M x 5 Lmoles of solute = 1.075 mol
Since glucose (C6H12O6) has a molar mass of approximately 180.16 g/mol, we can calculate the mass of glucose using the equation:
mass of solute = moles of solute x molar mass of solute
mass of glucose = 1.075 mol x 180.16 g/mol
mass of glucose = 194.0 g (rounded to three significant figures)
Therefore, approximately 194.0 grams of glucose (C6H12O6) would be required to prepare a 5000 mL solution with a concentration of 0.215 M. It's important to note that the molar mass of glucose used in this calculation may vary slightly depending on the level of precision required.
For more such questions on glucose visit:
https://brainly.com/question/397060
#SPJ8
in a land ecosytem , some organisms only live in the soil under rocks logs or plants . What would be a resonable prediction about how theses organisms would be affected if humans removed the coverings .
Answers
Answer:
The number of these organisms in the soil would decrease.
Explanation:
What is the only way to confirm that a chemical change has taken place?
O A. You can observe a color change.
B. You can see that the mass of material has changed.
C. You can record a temperature change.
D. You can tell that a new substance has formed.
Answers
Lead(Il) bromide is prepared using the following reaction:
Рb(NO3)2(aq) + KBr(aq) --> PbBr2(s) + KNO3(aq) (unbalanced)
How many milliliters of 1.25 M Lead(II) nitrate (Pb(NO3)2) are needed to prepare 150 grams of PbBr2 in excess potassium bromide solution. Assume you have 100% yield.
a) Fill in the stoichiometric flowchart below with the information from this problem.
b) Show the dimensional analysis calculation required o calculate the Millileters of 1.25M Lead (II) nitrate needed .
Answers
The volume of the lead II nitrate that is required is 328 mL.
What is the volume that is required?We know that we can be able to obtain the volume from the use of the stoichiometry of the reaction as we can see. We have to balance the reaction equation and then work from there.
Number of moles of the lead II bromide = 150 grams /367 g/mol = 0.41 moles
Given that;
1 mole of lead II nitrate produced 1 mole of lead II bromide
x moles of lead II nitrate produced 0.41 moles of lead II bromide
x = 1 mole * 0.41 moles / 1 mole
x = 0.41 moles
We now have;
number of moles = concentration * volume
volume = number of moles/concentration
volume = 0.41 moles/ 1.25 mol/L
volume = 0.328 L or 328 mL
Learn more about stoichiometry:https://brainly.com/question/9743981
#SPJ1
What is the structure of polypropylene
Answers
The Structure of polypropylene(C3H6)n is linear.
The chemical formula for polypropylene is (C3H6)n.It is a polymer with complex structure and also known as polypropene. Polypropylene is similar to polyethylene which belongs to polyolefin groups. Generally the polymers are synthesized from the polymerization process.What is polymerization?The process in which atom or small molecules joined together repeatedly to form a polymer with complex structure is called as polymerization.
The repeated unit of atom or small molecule is called monomer
Structure of polypropylenePolypropylene is synthesized from the polymerization of propylene which is the monomer unit.
It has a linear structure made of propylene hydrocarbon units.
Learn more about polypropylene on
https://brainly.com/question/20038733
#SPJ1

1. HF (aq) = H+ (aq) + F- (aq)
K 6.8 x 10-4
-
II. H₂C₂O4 (aq) = 2H+ (aq) + C₂0²-
K = 3.8 x 10-6
What is the K value for the
reaction below?
2HF (aq) + C₂02 (aq) =
2F (aq) + H₂C₂O4 (aq)
[?]
K = [?] x 10¹

Answers
Remember:
when you double moles: square the equilibrium constant
when you half the moles: Square root the equilibrium constant
when you reverse a reaction: take the reciprocal of the equilibrium constant

Which of the following contains the highest number of electrons?
OA) hydroxide ion
OB) oxonium ion
C) ammonium ion
OD) oxide ion
O E) all of them contains the same number of electrons
Answers
write the balanced equation for
[B]⁴[C][D]/[A]²