Answers
Answer:
It takes 5.83s to decrease the concentration of the reactant from 0.537M to 0.100M
Explanation:
A zero-order reaction follows the equation:
[A] = [A]₀ - kt
Where [A] is actual reaction of the reactant = 0.100M
[A]₀ the initial concentration = 0.537M
k is rate constant = 0.075Ms⁻¹
And t is time it takes:
0.100M = 0.537M -0.075Ms⁻¹t
-0.437M = -0.075Ms⁻¹t
5.83s = t
It takes 5.83s to decrease the concentration of the reactant from 0.537M to 0.100M
Related Questions
What is the volume of sodium hydroxide,
NaOH solution used to neutralised 25.00 mL
of 0.1500 M hydrocloric acid, HCI?
Answers
Answer:
sorry i don't know this question answeer
What is a reaction rate?
Answers
Answer:
A reaction rate is generally the speed (or rate) at which a chemical reaction takes place. It is usually expressed in terms of volume or a unit of time.
Answer:
the speed at which a chemical reaction takes place
A frog sitting at the edge of a water puddle. The mud and dirt surrounding the pond is labeled A, the frog is labeled B, and the water is labeled C.
Fill in the blank with the correct sphere label.
A:
geosphere
B:
biospere
C:
hydrosphere
Answers
Part A is labeled as geosphere, B is biospere and C is hydrosphere.
What are the different division of the earth?
The Earth can be divided into four interconnected spheres: the geosphere, atmosphere, hydrosphere, and biosphere.
The geosphere refers to the solid, rocky part of the Earth, including the crust, mantle, and core. The mud and dirt surrounding the water puddle would be considered part of the geosphere.
The biosphere includes all living things on Earth and the environments in which they live. The frog sitting at the edge of the water puddle is part of the biosphere.
The hydrosphere refers to all the water on Earth, including oceans, lakes, rivers, and groundwater. The water in the puddle would be part of the hydrosphere.
These spheres are interconnected and influence each other. For example, the biosphere relies on the geosphere for nutrients and minerals, while the geosphere is shaped by the movement of water in the hydrosphere.
Learn more about hydrosphere here: https://brainly.com/question/1699547
#SPJ1
At what temperature does Benzene boil when the external pressure is 440 torr
Answers
The boiling point of benzene when the external pressure is 440 torr is 80.1 °C (176.2 °F).
What is benzene?
Benzene is an organic compound composed of six carbon atoms in a ring structure, with one hydrogen atom attached to each carbon atom. It is a highly flammable, colorless liquid with a sweet smell. Benzene is a natural component of crude oil and is one of the most important organic chemicals used in industry. It is used in the production of plastics, resins, synthetic fibers, rubber, pesticides, detergents, drugs, and dyes. It is also used as a solvent for fats, oils, waxes, resins, and inks. Benzene is classified as a carcinogen and its exposure can cause a variety of adverse health effects, including acute myelogenous leukemia, lymphomas, and other forms of cancer.
To learn more about benzene
https://brainly.com/question/17211567
#SPJ1
What is the name of this formula unit? V₂S₃
Answers
Answer: Vanadium(III) Sulfide.
Explanation:
How much energy is required to heat 192 grams of lead from 18 degrees Celsius to 44
degrees Celsius?
Answers
The amount of energy required to heat the given amount of lead from 18°C to 44°C is 638.976 J.
The specific heat capacity of lead is 0.128 J/g°C.
To calculate the amount of heat energy required to heat 192 grams of lead from 18°C to 44°C, we can use the formula:
Q = m × c × ΔT
where:
Q = heat energy (in Joules)
m = mass of the substance (in grams)
c = specific heat capacity of the substance (in J/g°C)
ΔT = change in temperature (in °C)
Plugging in the given values:
m = 192 g
c = 0.128 J/g°C
ΔT = (44°C - 18°C) = 26°C
Q = 192 g × 0.128 J/g°C × 26°C
Q = 638.976 J
So, the amount of heat energy required to heat 192 grams of lead from 18°C to 44°C is 638.976 Joules.
To know more about Specific Heat Capacity:
https://brainly.com/question/27991746
what are minerals?
explained
Answers
A mineral is a substance such as tin, salt, or sulphur that is formed naturally in rocks and in the earth. Minerals are additionally observed in small portions in food and drink.
Why are minerals important?Minerals are integral for three essential reasons: building robust bones and teeth. controlling physique fluids inside and outdoor cells. turning the meals you devour into energy.
Why are minerals so important?Minerals are necessary for your body to remain healthy. Your physique uses minerals for many one of a kind jobs, which include maintaining your bones, muscles, heart, and talent working properly. Minerals are additionally necessary for making enzymes and hormones. There are two types of minerals: macrominerals and hint minerals.
Learn more about minerals here;
https://brainly.com/question/15844293
#SPJ1
de, it
this is a
your
Sample Response: This is a chemical change
because new substances are formed with different
properties and identities.
What did you include in your response? Check all that
apply
rmed with
This is a chemical change.
New substances are formed.
The properties and identities of the original
substances are changed.

Answers
If u click on all of them it gives u an A+ and doesn’t even read what u wrote. So u never really have to try hard on the writing parts just tell them u included them all
Answer:An image will not be formed.
Rays do not converge to or diverge from a common point.
/select all that apply
Explanation:
In using the Haber process in the formation of ammonia, what mass of hydrogen is needed to produce 51.0 grams of ammonia? 3 H₂(g) + N2 (g) → 2 NH3(g).
Answers
The mass of hydrogen needed to produce 51.0 grams of ammonia is ≈ 9.07 grams.
To determine the mass of hydrogen required to produce 51.0 grams of ammonia (NH3) using the Haber process, we need to calculate the stoichiometric ratio between hydrogen and ammonia.
From the balanced chemical equation:
3 H₂(g) + N₂(g) → 2 NH₃(g)
We can see that for every 3 moles of hydrogen (H₂), we obtain 2 moles of ammonia (NH₃).
First, we need to convert the given mass of ammonia (51.0 grams) to moles. The molar mass of NH₃ is 17.03 g/mol.
Number of moles of NH₃ = Mass / Molar mass
= 51.0 g / 17.03 g/mol
≈ 2.995 moles
Next, using the stoichiometric ratio, we can calculate the moles of hydrogen required.
Moles of H₂ = (Moles of NH₃ × Coefficient of H₂) / Coefficient of NH₃
= (2.995 moles × 3) / 2
≈ 4.493 moles
Finally, we can convert the moles of hydrogen to mass using the molar mass of hydrogen (2.02 g/mol).
Mass of H₂ = Moles × Molar mass
= 4.493 moles × 2.02 g/mol
≈ 9.07 grams
Therefore, approximately 9.07 grams of hydrogen is needed to produce 51.0 grams of ammonia in the Haber process.
Know more about the mass of hydrogen here:
https://brainly.com/question/14083730
#SPJ8
A change in tempo is the only thing that can make a musical cliché more effective.
A. False, a change in dynamics is the only thing that can make a musical cliché more effective.
B. True
C. False, using changes in tempo and dynamics can make a musical cliché more effective.
D. False, a change in tempo cannot make a musical cliché more effective.
Answers
False, using changes in tempo and dynamics can make a musical cliché more effective. Hence, option C is correct.
What is musical tempo ?Tempo in a music refers to pace of the music especially in high pitch sound waves. The most common unit of measurement for tempo is beats per minute (BPM), which is just the quantity of beats that may be captured in a minute at a certain pace.
A musical segment or composition is slower when the beats per minute is lower than when the BPM is greater. While a higher BPM is more likely to make the listener feel excited, a lower BPM is more likely to have a calming and/or sad effect on the listener.
Changing dynamics is some more effective way to make musical cliché. Hence, option C is correct.
Find more on musical tempo:
https://brainly.com/question/29574317
#SPJ1
__________ energy travels through matter in the form of waves.
Answers
Answer:
A wave is a form of energy therefore, it travels through matter in the form of packets of energy. Examples of waves include light, heat and sound waves. Matter from where the waves travel is known as a medium such as if waves travel through ocean then water is the medium.
Explanation: hope this helps
Which of these 2 elements will react similarly? *
Be, CI
Na, K
O Na, Mg
Na, CI
Answers
Answer:
Na, K
Explanation:
From the given choices, the two elements that will react similarly are Na and K.
This is because, Na and K are in the same group on the periodic table.
Na and K are both called Alkali metals and they have just one electron in their outermost shell.
The number of electrons in the outermost of an atom determines their chemical properties.
Since Na and K are in the same group, they chemically combine with other atoms in similar fashion.
Why do we fill aluminum cans with liquids (soda) then put them in the refrigerator
Answers
Answer:
hello hi what's up
Explanation:
ok bye
Answer:
to make it cold
Explanation:
Can anyone help me understand how to calculate the moles of H+ and OH-?

Answers
To calculate the moles of H+ and OH-, you need to know the concentration of the solution in terms of its pH or pOH value.
How to calculate the molesWhen you get the pH of the solution, you can use this formula to calculate the concentration of H+ ions: [H+] = 10^(-pH)
Also, if you know the pOH of the solution, you can use this formula to calculate the concentration of OH- ions: [OH-] = 10^(-pOH)
Having determined the concentration of H+ and OH- ions, the molarity formula can be used to calculate the number of moles of each ion as follows: moles = concentration (in mol/L) x volume (in L)
Learn more about moles calculation here:
https://brainly.com/question/14357742
#SPJ1
The solute in a colloidal system is alsoknown as the [?] phase.Bdispersedparticledispersionmedium
![The solute in a colloidal system is alsoknown as the [?] phase.Bdispersedparticledispersionmedium](https://i5t5.c14.e2-1.dev/h-images-qa/contents/attachments/yl50cQVuz0d3OG4CJ1Hn6df7W4A8LvIK.jpeg)
Answers
Explanation
Colloidal solutions, or colloidal suspensions, are nothing but a mixture in which the substances are regularly suspended in a fluid. A colloid is a mixture in which one substance is divided into minute particles (called colloidal particles) and dispersed throughout a second substance.
Hence, the solute in a colloidal system is also known as the dispersed particle phase
Calculate 0.039g of palladium to find the number of moles
Answers
The number of moles in Palladium is 0.00037 moles.
Given that,
Reacting mass of palladium = 0.039g
Molar mass of palladium = 106.42 g/mol
Therefore,
Number of moles = Reacting mass ÷ Molar mass
= 0.039 ÷ 106.42
= 0.00037 moles.
To learn more about calculating the number of moles, refer to:
https://brainly.com/question/14357742
https://brainly.com/question/15356425
For the reaction, CH4 + 2O2 -> 2H2O + CO2, calculate the percent yield of carbon dioxide if 1600. g of methane reacts with excess oxygen to produce 2200. g of carbon dioxide.
Answers
The percent yield of CO₂ In the reaction CH₄ + 2O₂ → 2H₂O + CO₂ if 1600g of methane reacts with excess oxygen to produce 2200 is 67.5%.
To calculate the percent yield of CO₂ in the given reaction, we need to first calculate the theoretical yield of CO₂. This can be done by stoichiometric calculations using the balanced chemical equation:
CH₄ + 2O₂ → 2H₂O + CO₂
The molar mass of CH₄ is 16.04 g/mol. Therefore, 1600 g of CH₄ is equivalent to 100 moles of CH₄. According to the stoichiometry of the reaction, 1 mole of CH₄ produces 1 mole of CO₂. Therefore, the theoretical yield of CO₂ is also 100 moles.
The molar mass of CO₂ is 44.01 g/mol. Therefore, the theoretical yield of CO₂ in grams is 4401 g.
The actual yield of CO₂ given in the problem is 2200 g.
Therefore, the percent yield of CO₂ is:
Percent yield = (actual yield / theoretical yield) x 100%
Percent yield = (2200 g / 4401 g) x 100%
Percent yield = 0.499 x 100%
Percent yield = 49.9%.
learn more about molar mass here:
https://brainly.com/question/22997914
#SPJ1
The value of H° for the reaction below is -72 kJ. ________ kJ of heat are released when 5.5 mol of HBr is formed in this reaction.
Answers
Answer:
-198 kJ
Explanation:
Hello there!
In this case, as the reaction is not given but we can see that HBr is formed, we can infer that reaction is:
\(H_2+Br_2\rightarrow 2HBr\)
Whereas the -72 kJ per moles of HBr are:
\(\frac{-72kJ}{2molHBr}\)
Thus, by multiplying by the formed 5.5 moles of HBr, the total energy release is:
\(\frac{-72kJ}{2molHBr} *5.5molHBr\\\\=-198kJ\)
Best regards!
ionic bonding: the transfer of electrons to form an ionic bond element no. of valence e- dot structure transfer of electrons ions formed compound formed name of compound na f mg o ba cl al o li p al n answers
Answers
Ionic bonding is a type of chemical bond that occurs when electrons are transferred between atoms to form ions. The resulting ions are attracted to each other by their opposite charges and form an ionic compound. Here is a summary of the information you provided:
Element: Na (sodium)
Number of valence electrons: 1
Dot structure: Na
Transfer of electrons: Na loses 1 electron to become Na+
Ions formed: Na+ and Cl-
Compound formed: Sodium chloride (NaCl)
Name of compound: Salt
Element: F (fluorine)
Number of valence electrons: 7
Dot structure: F
Transfer of electrons: F gains 1 electron to become F-
Ions formed: F- and Mg2+
Compound formed: Magnesium fluoride (MgF2)
Element: O (oxygen)
Number of valence electrons: 6
Dot structure: O
Transfer of electrons: O gains 2 electrons to become O2-
Ions formed: Ba2+ and O2-
Compound formed: Barium oxide (BaO)
Element: Li (lithium)
Number of valence electrons: 1
Dot structure: Li
Transfer of electrons: Li loses 1 electron to become Li+
Ions formed: Li+ and Cl-
Compound formed: Lithium chloride (LiCl)
Element: P (phosphorus)
Number of valence electrons: 5
Dot structure: P
Transfer of electrons: P gains 3 electrons to become P3-
Ions formed: Al3+ and P3-
Compound formed: Aluminum phosphide (AlP)
Element: N (nitrogen)
Number of valence electrons: 5
Dot structure: N
Transfer of electrons: N gains 3 electrons to become N3-
Ions formed: Al3+ and N3-
Compound formed: Aluminum nitride (AlN)
What is the correct equilibrium constant expression for the following reaction? 3A2 = 2B3 when the reaction started with the initial concentrations of A2 = 3 M and B3 = 2 M and continued until the equilibrium concentrations of A2 = 2.5 M and B3 = 2.5 M
Answers
Answer:
Kc = [B₃]²/[A₂]³ = 0.40
Explanation:
3A₂ ⇄ 2B₃
Given at equilibrium => [A₂] =2.5 and [B₃] = 2.5
Kc = [B₃]²/[A₂]³ = (2.5)²/(2.5)³ = (2.5)⁻¹ = 0.40
Select all answer choices that would result in units of moles.
a) RT/PV
b) PV/RT
c) mass ÷ molar mass
d) molar mass ÷ mass
e) molar mass × mass
f) molarity ÷ volume
g) volume ÷ molarity
h) molarity × volume
Answers
The correct options that will result in mole are option B, C, and H
How do i know which options will result in mole?To know the options that will result in mole, do the following:
Ideal gas law states as follow:
PV = nRT
Where
P is the pressure V is the volumen is the number of moleR is the gas constantT is the temperaturePV = nRT
Make n the subject by dividing both sides by RT
n = PV / RT (option B)
Mole, mass and molar mass are related according to the following formula:
Mole = mass / molar mass (option C)
Molarity is defined as mole per unit volume as shown below:
Molarity = mole / volume
Make mole the subject by cross multiplying.
Mole = Molarity × volume (option H)
Thus, from the above illustrations, we can conclude that the correct options which will result in mole are option B, C, and H
Learn more about mole:
https://brainly.com/question/30337259
#SPJ1
Convert 530 grams of Sodium carbonate (Na2CO3) into moles of Sodium carbonate (Na2CO3)
Enter your answer(s) here
Convert 4.2 moles of H2O into grams of H2O
Enter your answer(s) here
How many moles are in 12.04 x 10^23 particles of O2 ?
Enter your answer(s) here
How many particles are in 3 moles of magnesium (Mg)?
Answers
1. 5 moles
2. 75.6 grams
3. 2 moles
4. 1.806 x 10²⁴ particles
Further explanationGiven
mass and moles of compound
Required
mass, moles and number of particles
Solution
1. 530 g Na2CO3
mol = 530 g : 106 g/mol
mol = 5
2. 4.2 moles H2O
mass = 4.2 moles x 18 g/mol
mass = 75.6 grams
3. 12.04 x 10²³ O2
mol = 12.04 x 10²³ : 6.02 x 10²³
mol = 2
4. 3 moles of Mg
particles = 3 x 6.02 x 10²³
particles = 1.806 x 10²⁴
Determine the number of protons (p) and electrons (e) in Mg2.
A) 10 p, 12 e
B) 12 p, 12 e
C) 12 p. 10 e
D) 12 p. 14 e
E) 10 p, 10 e
Answers
Explain if the reaction of iron and copper sulfate supports the Law of Conservation of Mass. Use evidence and scientific reasoning to support your answer.
Answers
Endothermic and Exothermic Activity!! please help!!!

Answers
Chemical processes referred to as endothermic reactions occur when the reactants take in heat energy from their environment to create products.
What three things separate endothermic and exothermic states?Endothermic reactions quickly and efficiently take up heat-based energy from their surroundings. In contrast, an exothermic process transfers energy into the system's surroundings. A common illustration of an endothermic chemical reaction is photosynthesis.
Is water on the stove exothermic?Chemists refer to the process of boiling water as being endothermic since heat must be added. It follows that since certain processes need heat, others must also produce heat as they operate. They are referred to as exothermic.
To know more about endothermic reactions visit:-
brainly.com/question/23184814
#SPJ1
A sample of N2 has 1.70 moles and occupies 3.80 L at 25°C. How many moles are in a sample that occupies 1.45 L? Answer should be numbers only, no units, to 2 decimal places
Answers
The concept Avogadro's law is used here to determine the moles of gas present in the sample. Avogadro's law is also known as the Avogadro's principle or Avogadro's hypothesis. It is closely related to the ideal gas equation.
According to Avogadro's law, equal volumes of all gases under similar conditions of temperature and pressure contain equal number of molecules. It follows that the volume of the gas is directly proportional to the number of molecules.
The equation is:
V₁n₁ = V₂n₂
n₂ = V₁n₁ / V₂
3.80 × 1.70 / 1.45 = 4.45
To know more about Avogadro's law, visit;
https://brainly.com/question/3491421
#SPJ1
HOW MANY MOLES OF CS2 FORMS WHEN 2.7 MOLE C REACTS
Answers
HELP ME OUT PLS!!!!!!!!!
All stars begin their life in a
O black hole
O main sequence
O protostar
O nebula
Answers
Answer:
nebula
Explanation:
I am well informed
Solve this organic transformation....use - Br2,CCl4,KOH,CH3OH,Hg+2,diluted H2SO4, PCC,HBr,Mg,Dry ether,Na,H2,Pd,quinoline
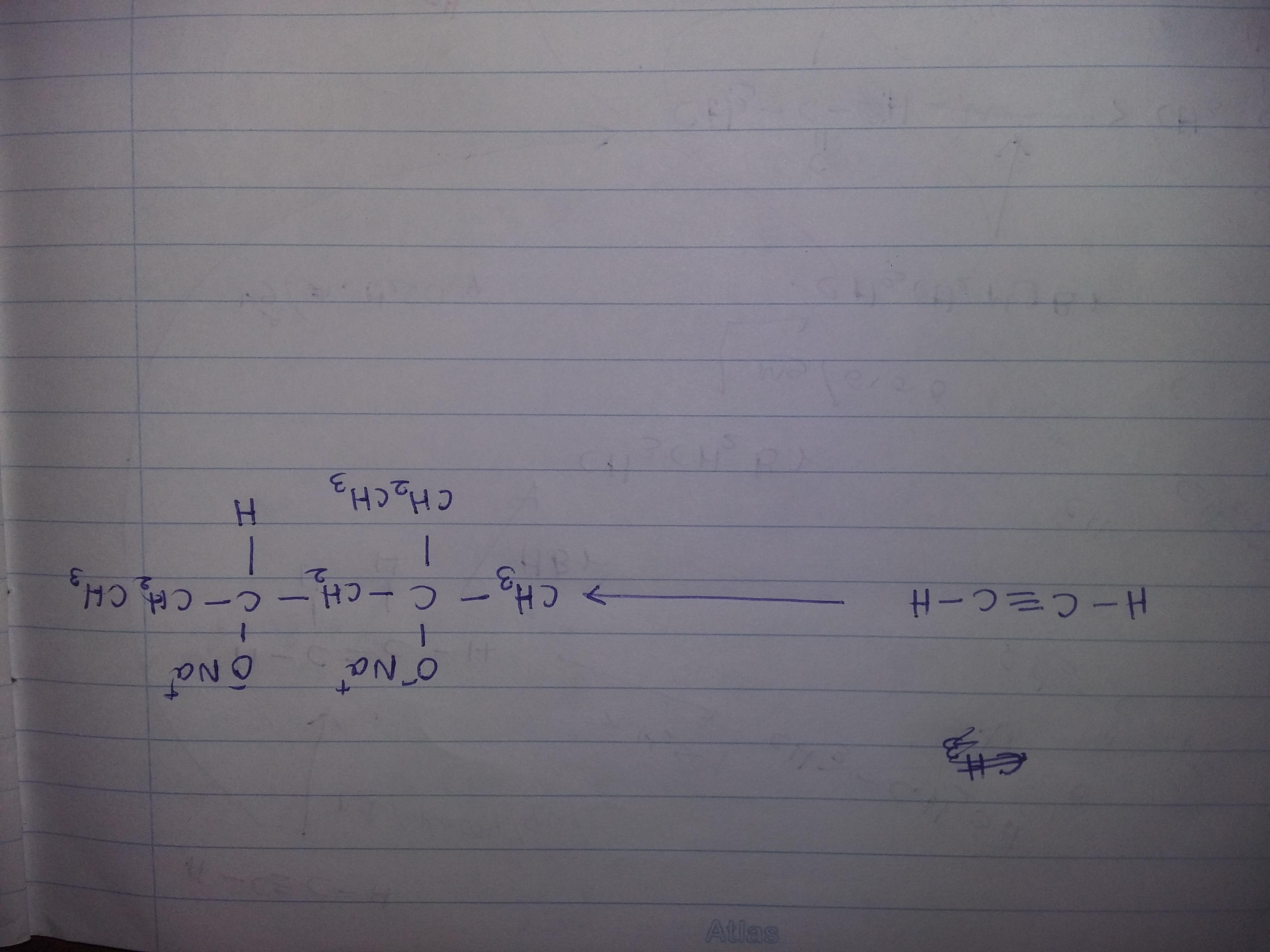
Answers
Organic transformation sequential equation using catalysts will be as follows:
2CH3-CH2-O => (alc. KOH) => CH2=CH2 + KCl + H2O => (Br2/CCl4) => CH2Br-CH2Br + Zn
CH2Br-CH2Br + Zn => (HBr /Pd) => CH2=CH2+ZnBr2
As can be visualized from above organic transformation equation, conversion of dry ether in presence of alkaline potassium hydroxide results in formation of unstable ethene. This dry unsaturated compound of ethene is stabilized by reaction that happens in presence of bromine or calcium tetrachloride as the catalyst which results in formation of ethylene bromide which in presence of highly efficient palladium as catalyst results in formation of stable ethene as byproduct. Thereby with formation of stable compound of ethylene, it releases zinc bromide as byproduct resulting completion of reaction equation. This stable product ethene is a double bonded carbon structure that is chemically extremely flammable and has planar structure.
To know more about organic transformation:
brainly.com/question/14413579
#SPJ1
Use the drop-down menus to rank the boiling points of the following hydrocarbons. Use a "1" to indicate the compound with the lowest boiling point. A carbon chain with 5 central carbons, with C H 3 bonded to the first, second, and last carbons in the chain. 2 central carbons with C H 3 bonded to the outside, and C H 3 bonded to the leftmost inside carbon. 2 central carbons, each with C H 3 bonded to them. A central C has H bonded left, above, behind to the right, and in front to the right.
Answers
The ranking of boiling point is based on the molecular weight and intermolecular forces between molecules.
2 central carbons, each with CH3 bonded to them. A central C has H bonded left, above, behind to the right, and in front to the right. A carbon chain with 5 central carbons, with CH3 bonded to the first, second, and last carbons in the chain. 2 central carbons with CH3 bonded to the outside, and CH3 bonded to the leftmost inside carbon.
In this case, all compounds are hydrocarbons, meaning they are non-polar molecules and exhibit van der Waals forces. However, the length of the carbon chain and the arrangement of atoms in the molecule affect the magnitude of these forces.
The first compound has only two carbons and exhibits weak intermolecular forces, so it has the lowest boiling point. The second compound has three carbons and a more complex arrangement of atoms, resulting in slightly stronger van der Waals forces and a higher boiling point.
The third compound has a longer carbon chain, which increases the molecular weight and results in stronger intermolecular forces, giving it a higher boiling point than the previous two. The fourth compound has the longest carbon chain and has multiple branches, which increases the surface area of the molecule and the strength of the intermolecular forces, giving it the highest boiling point.
Learn more about boiling point here:
https://brainly.com/question/28203474
#SPJ1
Answer:
The answers are 4, 2, 3, 1
Explanation:
